Unified Patent Court: state of play and what biotech companies can do to prepare now
The Unified Patent Court (UPC), an important new patent litigation system in Europe that will sit alongside existing national patent litigation in jurisdictions including the UK, will soon become a reality.
08.03.2023
First published in our Biotech Review of the Year publication (Issue 10).
This article provides an overview of the latest information, including key dates and updates on participating states, judges and divisions. We also outline the decisions that need to be made and implemented now by biotech companies in relation to patent portfolio management, litigation strategy and existing and future contracts in preparation for the UPC opening its doors later this year.
What is the UPC?
As many readers will be aware, the UPC is a new international court that is (finally!) due to open its doors later this year. In participating states, the UPC will have jurisdiction over disputes relating to the infringement and validity of patents granted by the European Patent Office (EPO) – both classic European patents and the new unitary patents that will become available when the UPC comes into force. However, during a transitional period of at least 7 years after the UPC begins, classic European patents may be opted out of the UPC system, with opt out becoming possible from the beginning of a sunrise period that will commence three months before the UPC comes into effect otherwise, both the UPC and national courts will have jurisdiction over these patents.
When will the UPC open its doors?
The start of the UPC was further postponed at the end of last year and the UPC roadmap at the time of writing foresees the sunrise period commencing on 1 March 2023 and the UPC opening its doors on 1 June 2023. Although this is only the latest in a number of delays, the postponement is said to be solely to give users of the UPC’s case management system (CMS) additional time to obtain the secure authentication systems required to interact with the CMS. No further postponements are therefore anticipated by the authorities, unless this or further issues relating to the CMS remain unresolved. The start date of the EPO’s transitional measures, which relate to the new unitary patent, was 1 January 2023. Steps that can be taken from 1 January 2023 and 1 March 2023 to prepare for the UPC are set out below (What should biotech companies be doing to prepare now?).
Which countries are participating in the UPC?
Not all European Patent Convention (EPC) states will participate in the UPC. Only those EPC states that (1) are EU Member States and (2) have signed and ratified the UPC Agreement (UPCA) may participate. At the time of writing:
- 17 EU Member States will participate in the UPC from the start: Austria, Belgium, Bulgaria, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Portugal, Slovenia and Sweden. Germany has not yet ratified the UPCA but has passed the legislation enabling it to ratify; Germany’s ratification instrument will be deposited with the EU Council to trigger the commencement of the sunrise period. The other 16 countries have already ratified the UPCA.
- 7 EU Member States have signed but have not yet ratified the UPCA: Cyprus, Czech Republic, Greece, Hungary, Ireland, Romania and Slovakia. The UPC will not have jurisdiction in these countries prior to ratification and unitary patents granted before the respective ratification of the country in question will have no effect in that country. Ireland’s ratification of the UPCA has been delayed by the need for a referendum to approve the associated amendment to its Constitution, however the Irish Government reaffirmed its commitment to participate last year and the referendum is expected to be held in 2023 or 2024.
- 3 EU Member States have not signed the UPCA and will not participate in the UPC: Croatia, Poland and Spain.
- A number of EPC states that are outside the EU will also not participate in the UPC, e.g. Albania, Iceland, Macedonia, Montenegro, Norway, Serbia, Switzerland, Turkey and the UK.
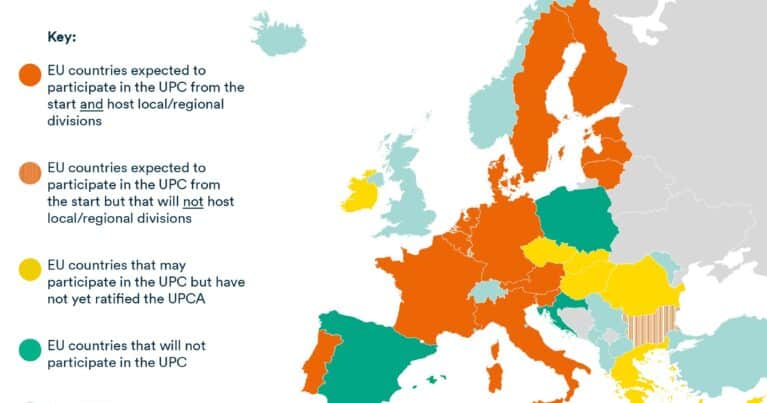
Where will the courts be located?
The UPC will be made up of a Court of First Instance, a Court of Appeal (which will hear appeals from the Court of First Instance) and a Registry (which will be responsible for administration of proceedings). The Court of Justice for the European Union will also have limited jurisdiction to hear questions on EU law referred to it by the Court of First Instance or Court of Appeal.
The Court of First Instance, made up of a Central Division and several local/regional divisions, will be spread across several locations (see below), whereas the Court of Appeal and the Registry will be located in Luxembourg.
Central Division of the Court of First Instance
The three seats of the Central Division were originally due to be in London (human necessities, chemistry and metallurgy, including pharmaceutical and biotech cases), Munich (mechanical engineering) and Paris (all other patent classifications). However, since the UK confirmed that it would no longer participate in the UPC post-Brexit, a new Central Division seat with responsibility for pharmaceutical and biotech cases has not been confirmed. The Italian Government has proposed Milan as the replacement for London but we understand that the final locations and responsibilities of the seats of the Central Division remain politically sensitive and may not be confirmed until the start date of the UPC.
Local and regional divisions of the Court of First Instance
Local divisions have been confirmed in Vienna, Brussels, Copenhagen, Helsinki, Paris, Düsseldorf, Hamburg, Mannheim, Munich, Milan, Lisbon, Ljubljana and The Hague and a Nordic-Baltic regional division based primarily in Stockholm will hear cases from Sweden, Estonia, Latvia and Lithuania. Three of the countries that will participate in the UPC from day 1 (Bulgaria, Luxembourg and Malta) will not host a local or regional division. However, additional local divisions may be added as further countries join the UPC, for example the Irish Government has announced that it will host a local division if it joins.
Who will the judges be?
The UPC Administrative Committee announced the appointment of 34 legally qualified judges (seven of whom have been appointed to the Court of Appeal) and 51 technically qualified judges in October 2022. The full list of judges is available from the UPC website: https://www.unified-patent-court.org/en.
Of the appointed judges, drawn from 13 of the 17 states that will participate in the UPC from the start, only five will be full time: the President of the Court of First Instance (Florence Butin), the President of the Court of Appeal (Klaus Grabinski) and three other Court of First Instance judges in the Presidium. All other judges will at least initially be part time.
The technically qualified judges who have been appointed are listed according to specific fields of technology. Eight judges have been appointed in the field of Biotechnology: Arwed Andreas Burrichter (Germany), Eric Enderlin (France), Rainer Friedrich (Germany), Paolo Gerli (Italy), Krister Karlsson (Finland), András Kupecz (the Netherlands), Roman Maksymiw (Germany) and Cornelis Schüller (the Netherlands). Of these, six are patent attorneys in private practice and one is an in-house patent attorney. It will therefore be interesting to see how potential conflicts of interest are managed given that these judges will continue in their private practice and industry roles while acting as judges part time.
What should biotech companies be doing to prepare now?
A number of decisions need to be made and implemented in the immediate run up to the UPC start date on 1 June 2023.
Opt outs
For patentees wanting to keep their patents and supplementary protection certificates (SPCs) out of the UPC during its early days, European patents and SPCs should be opted out during the sunrise period (from 1 March 2023) to avoid any day 1 challenges within the UPC. In this case, not only existing European patents should be opted out but also any expired European patents for which corresponding SPCs have been granted or for which future damages claims are contemplated.
Opt-out applications should be submitted via the online UPC CMS and must be made in respect of all states for which a European patent has been granted or that have been designated in the application (this includes EPC states that are not participating in the UPC, such as Spain and the UK, although the legal basis for requiring a proprietor to opt out European patents in non-UPC countries remains dubious). For co-owned patents, the agreement of all proprietors is required for opt out to be effective so discussions with co-owners should be held as soon as possible if this has not been done already. See below (Contracts) for further information on the steps to be taken for co-owned patents. Determination of the true proprietor of each designation of a European patent should also be completed, as the opt out needs to be filed by the true proprietor rather than the party registered as proprietor on the various national patent office registers (if different).
In order to access the CMS, a Client Authentication Certificate (which must be on a physical device such as a smart card or USB stick) and an Electronic Signature Certificate will need to be obtained. A list of providers that have claimed they meet the required technical standards for certification is available from the UPC website, including a number of providers that provide online rather than in-person identification. The certificates are specific to individual users so patentees wishing to opt patents out should consider carefully which individuals in their organisation will be responsible for opt outs and therefore need to obtain certification. Although the provider of the Client Authentication Certificate and Electronic Signature Certificate will need to be located within the EU, some will provide these to non-EU residents.
Any European patents that are not opted out before 1 June 2023 will automatically be subject to the jurisdiction of the UPC. However, such patents may be opted out at a later date if no action has been brought before the UPC in relation to that patent or associated SPC in the meantime. An opt out can also be subsequently withdrawn (once) so long as no action has been brought before a national court in relation to that patent or associated SPC prior to filing the withdrawal.
Unitary patents
Patentees also need to decide whether to request that current European patent applications are granted as unitary patents. Unlike classic European patents (which become a bundle of separate national rights on grant needing validation (where appropriate) and maintenance in each country), the new unitary patent will be a single patent that covers all participating UPC states at the time of grant and may not be opted out of the UPC. A single annual renewal fee will be payable. Assuming no additional countries ratify the UPCA before 1 June 2023, any unitary patents granted on that date will cover the 17 countries coloured orange on the map on the previous page.
For patentees who wish to obtain unitary patents, the EPO introduced two transitional measures on 1 January 2023:
- An applicant for a European patent that has reached the final phase of the grant procedure, i.e. for which a communication of intention to grant under Rule 71(3) EPC has been despatched, may file an early request for unitary effect before the start of the UPC so that a patent will have immediate unitary effect on grant, potentially even on day 1 of the UPC depending on timing.
- An applicant for a European patent coming to grant shortly before the UPC start date may request that the issue of the decision to grant a European patent be delayed until the start date so they do not miss the opportunity to obtain a unitary patent, which is only available for patents that grant on or after the UPC start date. In this case, the applicant will have one month from the start date to request unitary effect.
Litigation strategy
Clearly preparation for the UPC does not end with opt outs and requests for unitary patents.
For patents that are intended to be enforced within the UPC (European patents that are not opted out or unitary patents), decisions need to be taken as to where each patent should be enforced as there will often be a choice between a number of local, regional and central divisions under Article 33 UPCA. Factors relevant to this decision will include, for example, the likely approach of each division to bifurcation of validity and infringement proceedings, preliminary and final relief (including the security required for a preliminary injunction) and expert evidence, as well as the speed of judgment and quality of judicial decisions. Although many of these factors should become harmonised between divisions over time, there are expected to be national variations in procedure based on individual judges’ prior experience in the early years, so it is worth considering existing differences in national patent litigation in Europe and selecting a division based on the appointed judges.
Biotech companies should also be keeping a close eye on their competitors’ patents. For any European patents that are not opted out, 1 June 2023 could be the time to bring a central revocation action and take the opportunity to try to invalidate the patent in 17 jurisdictions at once. On the flip side, companies should be prepared to defend against any infringement actions brought by patentees within the UPC, where they will not have any choice over the division.
The UPC system is front-loaded and requires the case to be set out in detail from the beginning, including legal arguments, facts and evidence. A full defence must be filed by the defendant within three months of service of the claim, which does not leave long to put together a legal team, complete a statement of case and expert reports in an untested system. Further, any preliminary objection (such as a challenge to the jurisdiction of the UPC or the competence of the division selected by the claimant) must be lodged by the defendant within one month of service of the claim. For defendants who wish to delay proceedings within the UPC, there are likely to be a number of procedural challenges that could be made in the early days as judges and users of the system grapple with the rules, particularly some of the more opaque rules on jurisdiction. Given the short timeframes, it is worth biotech companies having some arguments up their sleeves now and potentially preparing draft written submissions that may be deployed quickly in the case of an early UPC challenge.
UPC litigation strategies should not be limited to consideration of the UPC itself. Since only 17 countries will participate in the UPC from the start and certain major European patent jurisdictions including the UK will never take part, there is ample scope for parallel litigation in the UPC and other European jurisdictions and a well-planned litigation strategy can make good use of the different systems. Given the fairly rigid fast-track procedure envisaged in the Rules of Procedure for the UPC, it is at present difficult to see how the UPC will be able to cater to some of the more complex litigation solutions available from national courts and, in light of the delays expected from procedural challenges in the UPC’s early days, a speedy reasoned judgment from a jurisdiction like the UK is likely to be extremely valuable. Pre-emptive strikes by filing national patent proceedings in participating UPC states before the UPC start date should also be considered where there is a concern that a competitor may have plans to file a central UPC action, in order to limit the jurisdiction of the UPC under the lis pendens rules of the Brussels Regulation.
Contracts
In order to formulate and implement a UPC strategy, patent owners and licensees should consider the terms of existing contracts and should also consider including UPC and unitary patent specific wording in future agreements.
- Opt-out decision making
Given the importance of a decision on whether or not to opt out a European patent and the consequences of that decision (the risk of central revocation in particular), it is important that parties ascertain who is entitled to decide whether to opt out. Under the UPCA an opt-out can only be exercised by the owner of the European patent. This means licensees will be reliant on the patent proprietor to opt out, even if contractually the licensee has control over the decision. Some agreements may address the UPC expressly, whereas others will not. If an agreement does not expressly address the UPC, determining who controls an opt-out decision will be a matter of interpretation of the relevant agreement. As an opt-out decision is a matter of jurisdiction relating to enforcement and defence of the patent, the enforcement and defence provisions, rather than prosecution provisions, are likely to be relevant.
If a European patent is jointly owned, an opt-out must be filed by all joint owners. This means that co-owners will need to agree on and implement an opt-out strategy. Parties should therefore examine the terms of any co-ownership agreement to determine whether it dictates how such decisions should be made, which party (if any) has control over opt-out decisions and whether there is a process for resolving disputes in the event of a disagreement.
The risk of central revocation of a European patent will be of particular concern to licensors as royalties will often be tied to validity of the licensed patents. Central revocation could therefore lead to a significant reduction or loss of royalty payments.
- Filing new patents in Europe
As outlined above, the unitary patent provides an additional option for patent applicants in Europe. Where an invention is the product of collaborative development, or a licence includes a European patent application, parties should consider who can decide whether to apply for a unitary patent, or to request that the licensed European patent application is granted with unitary effect. This decision will lie with the party that has the right to control patent prosecution. Parties will need to consider similar issues to those outlined above in relation to opt-out decisions. While in some cases it may be more cost effective to apply for a unitary patent rather than national patents (although this will depend on many factors), the risk of central revocation is still likely to be a concern for both licensors and licensees.
- Future agreements
Parties negotiating licence and collaboration agreements should agree a strategy for dealing with European patents. Parties should consider who will have control over opt-out decisions, under which circumstances an opt-out can be withdrawn and whether to agree a default opt-out position. If control over those decisions can be exercised by the licensee (or one of the co-owners) that party should consider ensuring the licensor (or its fellow co-owner) is obliged to comply with its decision and not opt out or withdraw an opt-out without the licensee’s consent.
Parties should also consider now whether they need to alter their stance on control of patent prosecution in light of the new unitary patent. Licensors may in the future wish to include express provisions requiring a licensee with control of prosecution to seek the licensor’s approval or input on decisions as to whether or not a licensed European patent application is granted as a unitary patent. Licensees may also wish to require some level of cooperation on decisions whether to seek a unitary patent. Exclusive licensees in particular may wish to require their preferred approach to be followed, particularly if the licensor is controlling the patent prosecution, and step-in rights if the licensor decides to let a unitary patent lapse at a later date.
Finally, parties may also wish to consider the input they have into the formalities of the application process. In particular, depending on where co-owners are resident or have a place of business, the governing law of a co-owned unitary patent as an item of property may differ depending on which co-owner is listed first as applicant. An administrative decision can therefore have important ramifications (although ultimately, provided that law allows the parties to regulate their respective rights by contract, co-owners are best advised to vary the default legal position anyway so that the arrangements are more suitably tailored to reflect their wishes).

Gregory Bacon
Author

Charlie French
Author

Ellen Lambrix
Author
