Spotlight on mRNA – What is mRNA and what can it be used for?
mRNA: a new era in genetic medicine?
24.09.2021
Last month, August 1st, marked annual RNA Day; a celebration of ribonucleic acid – one of the four major types of macromolecules essential to all forms of life. However, before the COVID-19 pandemic, the terms “RNA” and “mRNA” were not part of the general public lexicon. Almost overnight that all changed with the approval[1] by regulators of COVID-19 vaccines from Pfizer/BioNTech and Moderna (both of which use mRNA technology). It took Moderna just two days from release of the virus sequence to finalise the sequence for its COVID vaccine and only a further 25 days to complete manufacture of the first clinical batch, an incredible feat given that usual vaccine development timescales are measured in years. The approval of the Pfizer/BioNTech and Moderna mRNA COVID vaccines marks the first time that in vitro transcribed mRNA has been approved for use in humans.
Now, as we look to a future beyond the pandemic, mRNA is justifiably enjoying a moment in the limelight. It has moved from being viewed as a speculative research area to becoming a key asset in leading pharma and biotech pipelines. This new platform is being talked about as having transformational potential, not only as a vaccine platform but also for use as a therapy against genetic diseases, in cancer immunotherapy and in regenerative medicine. If the hype is to be believed, the possibilities are enormous.
In this second article of our five part series, we take a closer look at mRNA, what it is and what it could potentially be used for. In later instalments we will take a look at the intellectual property landscape, some of the recent mRNA deal and partnership activity and examine how mRNA vaccines and therapies are regulated.
The basics: what is mRNA?
mRNA stands for “messenger ribonucleic acid” or “messenger RNA”. It is one of several types of RNA molecules including tRNA (transfer RNA) and rRNA (ribosomal RNA).
To understand mRNA it helps to start with some basics. Most people are quite familiar with the concept of DNA. At a very basic level, DNA contains our genetic code and is located in the nucleus of our cells. It is a double stranded molecule made up of a sugar phosphate backbone and four nucleotide bases (adenine (A), guanine (G), cytosine (C), and (T), thymine). The sequence of these nucleotide bases in DNA encodes our genes which are themselves the blueprints for making the thousands of proteins that are needed for life. In structural terms, RNA is a molecule similar to DNA in that it is made up of a sugar phosphate backbone (albeit with one extra oxygen atom) and four nucleotide bases (ACGU instead of ACGT where the U stands for uracil).
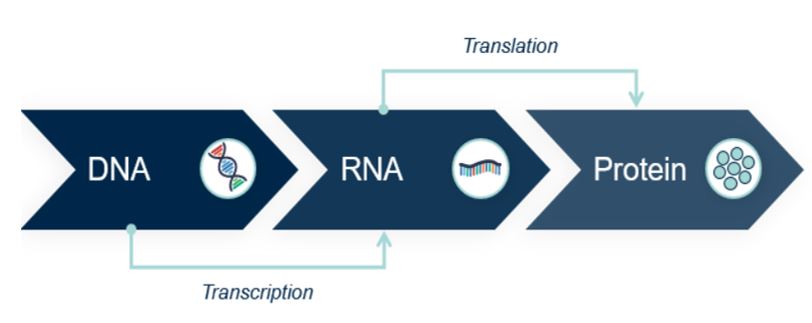
mRNA plays a starring role in what has become known as the “central dogma” of molecular biology (a term coined by Francis Crick in 1958, five years after deciphering the structure of DNA with James Watson). In simplistic terms, the central dogma describes how the genetic information coded for by our DNA is transcribed (i.e. copied) into molecules of mRNA. mRNA then carries this code for synthesizing a particular protein or proteins to our ribosomes (i.e. it acts as a messenger, hence the name). Ribosomes act like small factories manufacturing proteins; they take the mRNA code, translate it and produce the coded for proteins. mRNA is therefore essential to life; without it, the genetic code of our DNA would never make it out of the cell nucleus to be translated into the thousands of proteins we need to live.
The history of mRNA research is interesting in itself and we’ve looked at this in a separate article (“Spotlight on mRNA – A brief history of mRNA research”). From its discovery in 1961 the path to approval of mRNA vaccines has been marked by many challenges. It wasn’t until a crucial discovery was made in the mid-2000s by the winners of the 2022 Breakthrough Prize in Life Sciences, Katalin Karikó and Drew Weissman, that the theoretical possibilities of using mRNA started to become a reality.
Potential uses of synthetic mRNA
The opportunities for using synthetic mRNA to prevent or treat diseases appear to be vast. The technology can be viewed as a platform. If you have the genetic sequence of the protein or antigen you want to produce in the body, it is relatively quick and simple to design and synthesise an mRNA which codes for that molecule. In that sense it’s been described by some commentators as a “plug and play” technology. In very simple terms, the delivery system stays broadly the same and the only thing that needs to change between different mRNA therapies is the coding region of the mRNA. This speed of design and scalability of manufacturing which is one of the major advantages for mRNA over other vaccines and therapies. Admittedly this is an oversimplification as delivery system design is crucial will vary depending on route of administration and the intended target of the mRNA, however this does not undermine the platform nature of the technology.
A further advantage that mRNA may offer over other more traditional vaccine types (such as live attenuated vaccines, inactivated vaccines and sub-unit vaccines) and over DNA based vaccines is safety. mRNA is degraded in the body by normal cellular processes which reduces the risks of metabolite toxicity (i.e. the formation of toxic intermediate compounds or end products during the degradation process). In addition, mRNA is non-infectious and non-integrating (i.e. it does not cause DNA insertions) which gives it a further advantage over attenuated and DNA based vaccines[2].
We have detailed some of the potential uses which are currently under development below.
Vaccines for infectious disease: Given the success of the mRNA based COVID vaccines, an obvious next step is to expand the use of prophylactic mRNA vaccines for other infectious diseases. This is precisely what vaccine companies the world over racing to do. Influenza is an obvious target and a number of companies are working on mRNA flu vaccines. Beyond COVID-19 and flu, there are a number of other mRNA vaccines currently under development for a variety of indications including cytomegalovirus (CMV), zika virus, respiratory syncytial virus (RSV), Epstein-Barr virus (EBV), rabies, yellow fever, rotavirus and malaria. Such is the excitement about the potential of mRNA there are numerous companies working in this space including Moderna, Pfizer, BioNTech, CureVac, GSK, Sanofi, Translate Bio and Arcturus Therapeutics, to name just a few.
Treatment of genetic diseases: there has been a good deal of excitement about the potential of mRNA as a protein replacement therapy to treat certain rare genetic diseases and it is easy to see why. Many rare genetic diseases are caused by defects in or deficits of proteins. In theory mRNAs could be designed to trigger the expression of those missing proteins, or healthy versions of the defective proteins in a patient’s body. Research is already underway on the use of mRNA as a therapy to treat various inherited metabolic diseases which are often life-threatening or disabling. Moderna in particular has several therapies for various rare liver diseases in its pipeline. Treatment for these disorders would involve an intravenous delivery of mRNA which would stimulate production of therapeutic proteins in the liver. Several other biotech companies are also working on inherited disorders including Translate Bio and Arcturus who are both working on cystic fibrosis treatments. Both the Arcturus and Translate Bio therapies involve delivery of an aerosolized form of mRNA directly to lung cells. The delivered mRNA contains instructions for the production of CFTR protein which is either missing or defective in CF patients.
One distinct advantage of mRNA therapy is that the mRNA never enters the nucleus of the cell and therefore does not edit a patient’s DNA. The downside of this however is that mRNA therapy would not be a one off treatment and the mRNA would need to continue to be administered at intervals.
Cancer immunotherapy: Immunotherapy involves harnessing the immune system to target cancer cells in the same way that the immune system targets infection. There are a number of different types of mRNA immunotherapy currently under development. One such approach involves the development of personalised cancer vaccines. This involves using genetic sequencing to identify mutations on a patient’s cancer cells called neoepitopes. An mRNA vaccine is developed which encodes for each of these neoepitopes which can help the immune system distinguish the cancer cells from normal cells. Once the mRNA is injected into the patient, the patient’s cells will then express the neoepitopes with the goal that this will help the patient’s immune system recognise the cancer cells as foreign and attack them.
Another approach under development involves administering the mRNA locally at the site of a tumour. The administered mRNA encodes for potent proteins which stimulate an anti-cancer T cell immune response at the site of the tumour.
Clinical trials are currently underway using mRNA to treat various types of cancer including, lymphoma, skin cancer, ovarian cancer, prostate cancer, breast cancer, non-small cell lung cancers, gastrointestinal cancer, and pancreatic cancer, among others.
Regenerative therapeutics: mRNA therapy is also being tested to promote the growth of new blood vessels. In particular, Moderna and AstraZeneca are collaborating on an mRNA based treatment for myocardial ischemia (a type of heart disease which occurs when blood flow to the heart is reduced, preventing the heart muscle from receiving enough oxygen). The therapy uses a locally administered mRNA which encodes for the protein VEGF-A. VEGF-A is a growth factor which promotes the growth of new blood vessels and pre-clinical data has suggested that expression of VEGF-A in the heart could increase blood flow and partially restore cardiac function. Arcturus Therapeutics is also testing mRNA therapies for heart disease.
Others: as is clear, there are numerous, diverse ways to harness the power of mRNA to prevent or treat disease. However the various uses for mRNA detailed above are by no means an exhaustive list. In addition to the uses mentioned above there are also vaccines and therapies under development for the prevention and treatment of HIV and tuberculosis and the treatment of various autoimmune conditions.
Our take
The field of research into the use of mRNA to prevent and treat a variety of diseases has a long history and the technology has potential application in many different indications. Recent technological advances and approval of the first in vitro transcribed mRNA vaccines has catapulted this new drug class into the spotlight and the field is enjoying exceptional growth and an in-pouring of investment. It seems possible that we are standing on the precipice of a promising new era of mRNA-based medicine and we are excited to watch as the technology develops and the possibilities unfold.
In the next articles of this series, we will examine the mRNA intellectual property landscape, recent deals and partnerships and review how mRNA vaccines and therapies are regulated.
—————
[1]Both the Pfizer/BioNTech and Moderna vaccines were initially approved for use in the UK under an Emergency Use Authorisation and in the United States under an Emergency Use Authorisation. The Pfizer/BioNTech vaccine received full approval in the United States in August 2021
[2] Pardi, N., Hogan, M., Porter, F. et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018). https://doi.org/10.1038/nrd.2017.243

Ellen Lambrix
Author
